- Mastering Zeta Potential: Theory and Measurement Tutorial
- Mastering Zeta Potential: Insights on Measurement and Theory
- Mastering Zeta Potential: Real World Considerations
- Mastering Zeta Potential: Before You Start
- Mastering Zeta Potential: Understanding Its Importance
- Mastering Zeta Potential: Electrostatic Repulsion
- Mastering Zeta Potential: Understanding Double Layers
- Mastering Zeta Potential: Role of Electrolyte Concentration
 Understanding the Basics of Colloidal Interactions
Understanding the Basics of Colloidal Interactions
In the realm of colloid chemistry, understanding the interactions between particles is pivotal. These interactions are largely governed by the zeta potential, a key indicator of the stability of colloidal dispersions. Zeta potential is essentially the electric potential in the interfacial double layer at the location of the slipping plane versus a point in the bulk fluid away from the interface.
The Role of Electrolyte Concentration
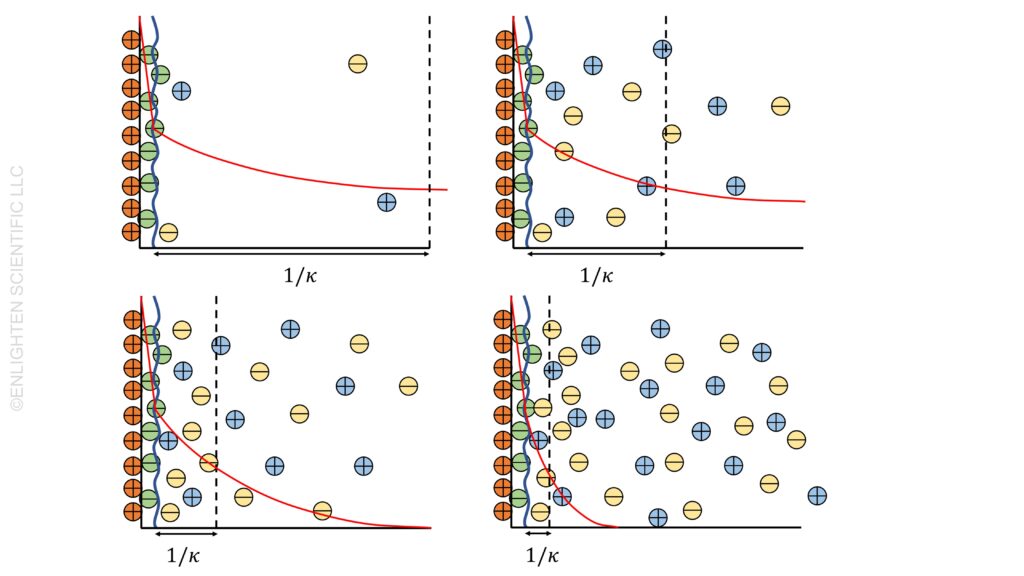
The concentration of electrolytes in a solution significantly impacts the double layer’s thickness around colloidal particles. Generally, an increase in electrolyte concentration leads to a decrease in double layer thickness. This phenomenon can be explained by the Debye-Hückel theory, which describes how ions in the electrolyte screen the surface charge of the particles, thus affecting the zeta potential.
Observing the Decay of the Double Layer
With a dilute electrolyte, the decay of the double layer is slow, indicating a thicker double layer and higher repulsion between particles. This thicker double layer can be attributed to lower ionic strength, I, in the solution. As a result, the zeta potential is higher, contributing to greater colloidal stability.
 Effects of High Ionic Strength
Effects of High Ionic Strength
Conversely, at high salt concentrations, we observe a thinning of the double layer. The ionic strength, which is a measure of the concentration of all ionic species (taking into account their valencies), plays a crucial role here. Polyvalent ions, due to their higher valency, are more effective at screening than monovalent ions, thus reducing the double layer thickness more efficiently.
Balancing Attraction and Repulsion in Colloids
In colloidal systems, understanding the balance between attraction and repulsion is essential. The thinning of the double layer at high electrolyte concentrations leads to a decrease in repulsion between particles. This can lead to a “collapsed” state of the double layer, indicating reduced stability and increased risk of aggregation or flocculation.
Conclusion: The Critical Role of Zeta Potential
In summary, the effect of electrolyte concentration on the zeta potential and double layer thickness is a fundamental aspect of colloidal chemistry. It plays a critical role in predicting and controlling the stability of colloidal systems. Understanding these interactions helps in various applications, from industrial processes to pharmaceutical formulations, where controlling the balance of colloidal forces is essential.
 Understanding the Basics of Colloidal Interactions
Understanding the Basics of Colloidal Interactions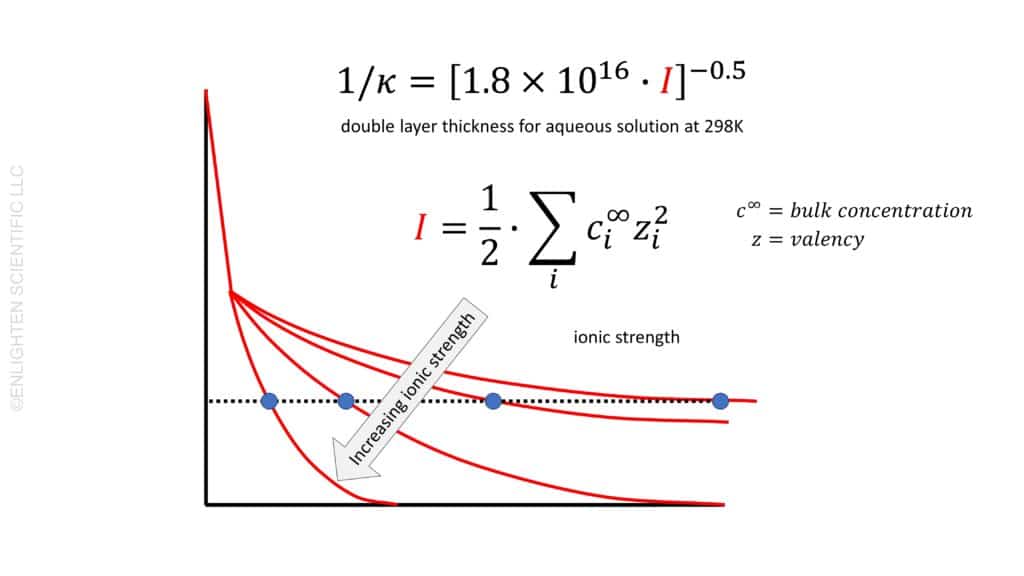 Effects of High Ionic Strength
Effects of High Ionic Strength