Key Role of Electrode Materials in Zeta Potential Measurement
Zeta potential is a crucial parameter in understanding the stability of colloidal systems, and its accurate measurement is especially challenging in high salt/high conductivity conditions where the choice of electrode material is often overlooked. This study focuses on how different materials – palladium (Pd), platinum (Pt), and platinum black (Pt black) – impact measurements, particularly in high potassium chloride (KCl) environments. I also delve into the observation of particle formation, a crucial aspect in these measurements.
Electrode Material and Electrophoretic Mobility
My findings indicate that in high salt/high conductivity conditions, the choice of material significantly affects the electrophoretic mobility, a direct measure of zeta potential. Pt black delivers the most consistent and reliable data, while Pd and Pt exhibit variations in data accuracy. This variation becomes increasingly pronounced with higher [KCl] concentrations, underscoring the importance of choosing the right electrode material for precise zeta potential measurements.
Particle Formation in High Salt Solutions
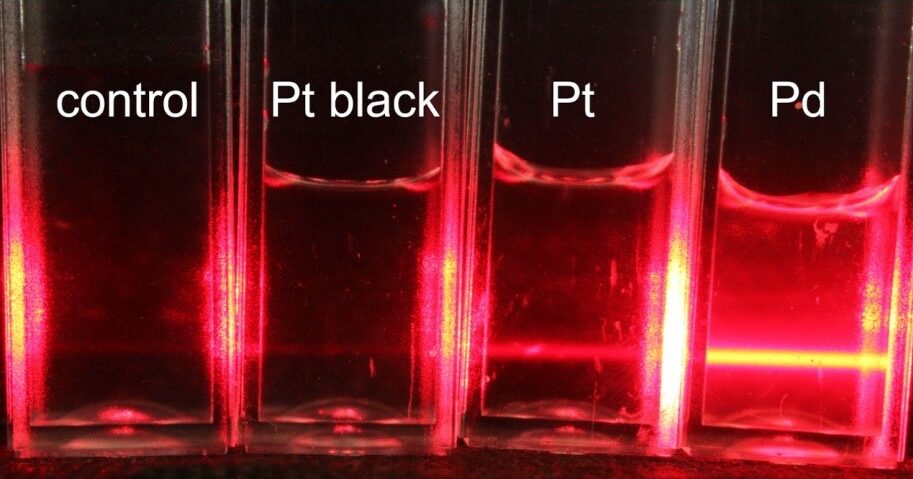
A notable observation in my study is the formation of particles during electrophoretic measurements, particularly with Pd and Pt. This phenomenon is critical as it can influence the validity of the experimental results. In high [KCl] solutions, Pd not only showed significant changes in electrophoretic mobility but also led to the formation of colloidal particles. These particles, resulting from electrode degradation due to electrolysis, can skew the light scattering measurements, thereby affecting the accuracy of zeta potential determination.
Visual Assessment of Electrodes Post-Experiment
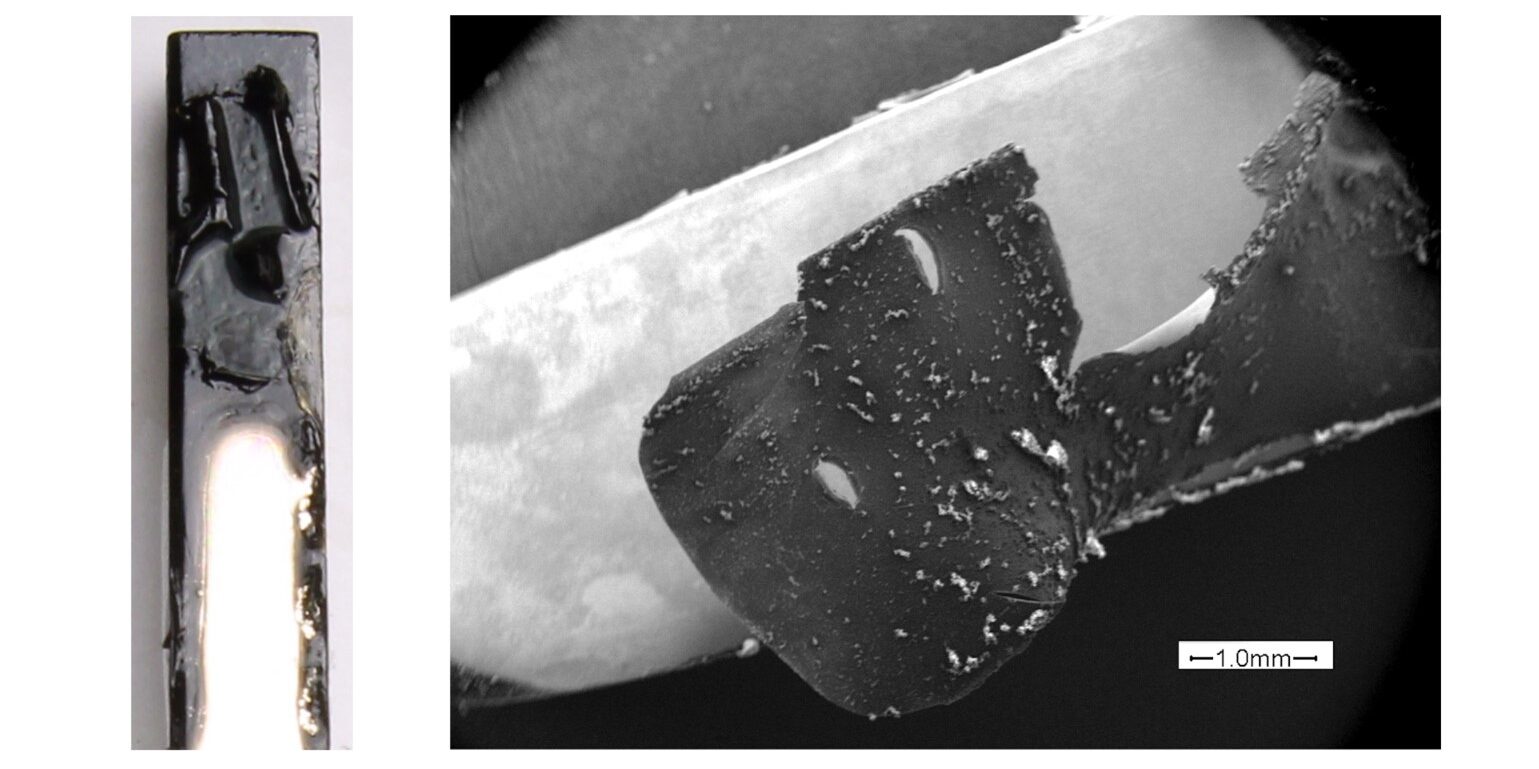
The physical condition of electrodes after experiments in high salt solutions provided additional insights. While Pt black maintained its structural integrity, Pd exhibited noticeable degradation and discoloration, indicating their unsuitability in high salt/high conductivity conditions. This degradation is directly linked to the observed particle formation and can significantly compromise the reliability of zeta potential measurements.
Conclusion: Optimal Electrode Selection for Accurate Zeta Potential Analysis
My comprehensive analysis emphasizes the importance of electrode selection in zeta potential measurement under high salt/high conductivity conditions. Pt black not only ensures more accurate readings but also mitigate the issue of particle formation seen with Pd and Pt. This finding is vital for researchers and practitioners in the field, advocating for the use of Pt black to enhance the reliability and accuracy of colloidal stability assessments in high salt/high conductivity environments.