- Mastering Zeta Potential: Theory and Measurement Tutorial
- Mastering Zeta Potential: Insights on Measurement and Theory
- Mastering Zeta Potential: Real World Considerations
- Mastering Zeta Potential: Before You Start
- Mastering Zeta Potential: Understanding Its Importance
- Mastering Zeta Potential: Electrostatic Repulsion
- Mastering Zeta Potential: Understanding Double Layers
- Mastering Zeta Potential: Role of Electrolyte Concentration
 Introduction to Electrical Potential in Particle Double Layers
Introduction to Electrical Potential in Particle Double Layers
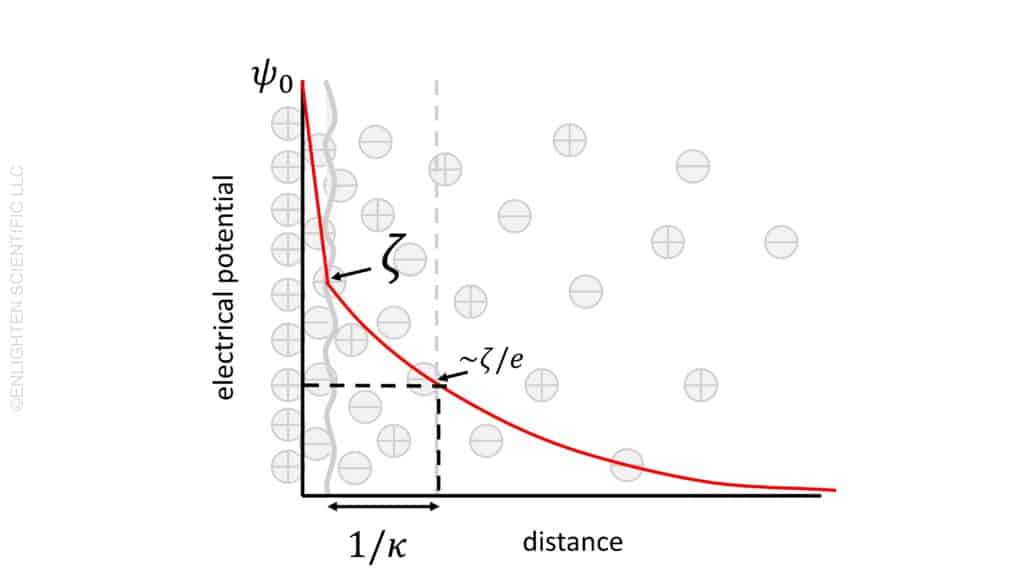
The concept of electrical potential plays a pivotal role in the understanding of double layers around charged particles. In simple terms, electrical potential represents the energy required to move a charge to a specific point from an infinitely distant location. This energy, often expressed in volts, indicates the level of repulsion between charged particles based on their separation.
The Starting Potential: ψ0
At the surface of a particle, the electrical potential begins at a value referred to as ψ0. This value sets the baseline for understanding the changes in potential as one moves away from the particle surface. The presence of a charged particle, carrying a specific number of elemental charges, creates a zone of influence where the potential is distinctly measurable.
Screening by Adsorbed Material
As we move away from the particle surface, the initial surface charge encounters a screening effect. This is caused by the adsorption of material with an opposite charge. This phenomenon leads to a decrease in potential between the particle surface and what is known as the slipping plane. The interaction between the particle’s surface charge and the adsorbed material is crucial in understanding the behavior of the double layer.
Zeta Potential at the Slipping Plane
The potential at the slipping plane holds significant importance and is defined as the zeta potential, ζ. This value is critical in understanding the stability and behavior of colloidal dispersions. Zeta potential offers insight into the degree of repulsion or attraction between particles in a suspension, influencing factors like aggregation, sedimentation, and overall stability.
Beyond the Slipping Plane: Influence of Electrolytes
Moving further from the slipping plane, the potential continues to decrease. This decrease is influenced by the presence of electrolytes and the polar nature of the liquid. The interaction between the charged particles and the surrounding medium, including the ions present in the electrolyte, shapes the extent and nature of the potential decrease.
Defining the Thickness of the Double Layer
A key concept in understanding the double layer is its “thickness.” This thickness is defined as the distance at which the potential decays to 1/e (approximately 37%) of its initial value at the particle surface. Mathematically, this distance is represented as 1/κ. Various factors, including the concentration of ions in the medium and the nature of the particle surface, influence this thickness.
Conclusion
The exploration of electrical potential in particle double layers reveals the intricate balance of forces at play at the microscopic level. Understanding these concepts is essential in fields like colloidal chemistry, material science, and nanotechnology, where the behavior of particles under different conditions can have significant implications. The electrical potential serves as a guide to the interactions and stability of particles in various environments.
 Introduction to Electrical Potential in Particle Double Layers
Introduction to Electrical Potential in Particle Double Layers