- Mastering Zeta Potential: Theory and Measurement Tutorial
- Mastering Zeta Potential: Insights on Measurement and Theory
- Mastering Zeta Potential: Real World Considerations
- Mastering Zeta Potential: Before You Start
- Mastering Zeta Potential: Understanding Its Importance
- Mastering Zeta Potential: Electrostatic Repulsion
- Mastering Zeta Potential: Understanding Double Layers
- Mastering Zeta Potential: Role of Electrolyte Concentration
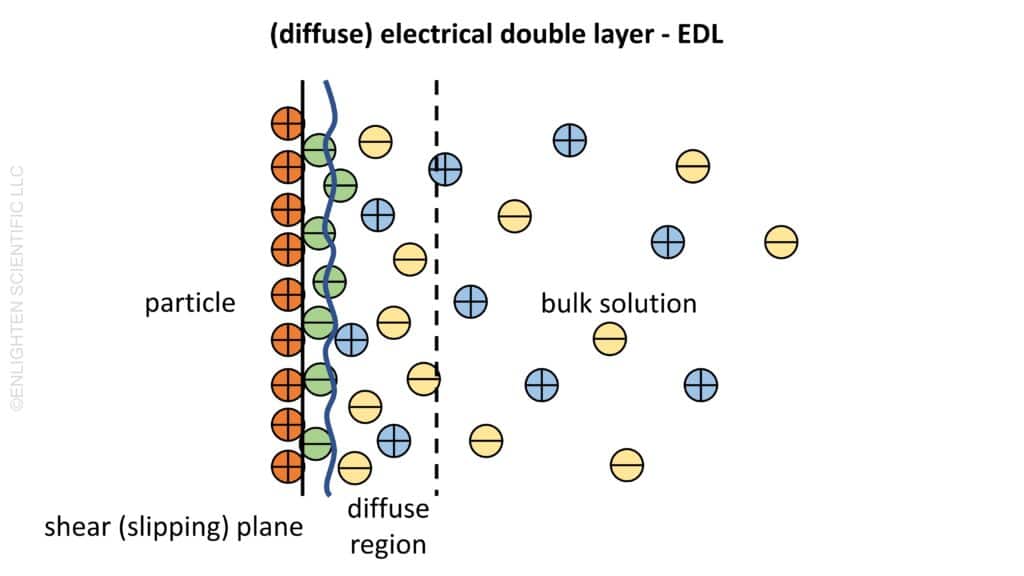
The Formation of the Electrical Double Layer
At the heart of this interaction is the charged particle surface. In a typical scenario, the surface of the particle carries a positive charge. To maintain electrical neutrality, this charge is balanced by a concentration of opposite charges in the surrounding medium, often in the form of negatively charged ions (anions) adsorbed onto the particle surface. This adsorbed layer is known as the rigid or Stern layer, characterized by ions that are strongly associated with the particle.
In the bulk of the electrolyte solution, there is a uniform distribution of both anions and cations. However, near the positively charged particle, anions are attracted towards the surface, creating a region of higher anion concentration. Conversely, there is a repulsion of cations (positively charged ions), resulting in a lower concentration of these ions near the particle. This forms what is known as the diffuse layer, comprising ions that are not as tightly bound to the particle as those in the rigid layer.
The combination of the rigid layer and the diffuse layer constitutes the diffuse electrical double layer. This double layer is crucial in understanding the electrostatic properties of the particle in the solution.
Transition to Bulk Solution and the Shear Plane
As we move away from the particle surface, there is a transition from the region dominated by the diffuse layer, with an excess of counterions, to the bulk solution, which has a uniform concentration of ions. The point at which this transition occurs is known as the shear or slipping plane. This plane is significant because it demarcates the boundary between material that is either adsorbed or strongly associated with the particle (and thus moves with the particle) and the free liquid.
Quantifying Electrostatic Repulsion Between Particles
Understanding the magnitude of electrostatic repulsion between such particles is complex. As two particles with similar charges approach each other, their respective electrical double layers begin to interact. The closer they get, the more the outer diffuse layers overlap, leading to an increase in electrostatic repulsion. This repulsion can be quantified using various theoretical models, one of the most prominent being the DLVO (Derjaguin, Landau, Verwey, and Overbeek) theory. This theory combines the effects of electrostatic repulsion (as described above) and van der Waals attraction to predict the overall interaction between particles.
Spatially, the electrostatic repulsion decreases with increasing distance from the particle surface. The intensity of this repulsion is not only a function of the distance but also depends on factors like the ionic strength of the solution, the size of the ions, and the surface charge density of the particles.
In summary, the behavior of charged particles in an electrolyte solution, especially in terms of electrostatic repulsion, is a nuanced topic that requires a deep understanding of the electrical double layer and its interactions. It’s a field rich with both theoretical and practical applications in chemistry, physics, and material science.